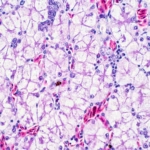
A recent single-cell RNA sequencing (scRNA-seq) study explains why some skin scars, such as keloids, become excessively thick and hard. This research, published by Yue Li and colleagues in Scientific Reports, identified distinct cell types within fibrotic skin. Most importantly, it pinpointed the CLOCK protein—a molecular switch—as a critical factor in severe scarring.
Fibrosis is a major global health concern. It involves the abnormal buildup of the extracellular matrix (ECM), the material that gives structure to tissue. In this process, the excessive proliferation of fibroblasts the primary repair cells—drives disease progression. While fibrosis is necessary for healing, excessive fibrosis leads to scar thickening, organ hardening, and chronic disease.
However, effective treatments remain elusive because the underlying cellular mechanisms are still poorly understood.
Researchers used scRNA-seq (single-cell RNA sequencing) to closely examine fibroblasts in various skin conditions. scRNA-seq is a powerful method that lets scientists analyze gene activity in thousands of individual cells. They studied cells from normal skin, typical scars, keloids, and scleroderma. Keloids are aggressive scars that grow beyond the original wound. Scleroderma is a disease causing chronic skin hardening.
- Distinct Cell Subpopulations: Analysis showed that fibroblasts are not uniform. Distinct subpopulations of fibroblasts exist, and each is unique to its specific fibrotic condition.
- Pivotal Regulators Identified: The study crucially identified a unique “master switch” or pivotal regulator for the fibroblasts in each condition:
- IRF4 for typical scars
- CLOCK for keloids
- RUNX3 for scleroderma
- HOXC4 for normal skin
The CLOCK gene (Circadian Locomotor Output Cycles Kaput) proved to be the most significant finding. This gene usually regulates the body’s internal clock. The research found that keloid tissues predominantly expressed CLOCK.
Further lab experiments confirmed CLOCK’s essential role:
- Enhanced Activity: Increased activity (upregulation) of CLOCK significantly boosted fibroblast proliferation and migration in vitro (in a dish). This suggests CLOCK acts like an accelerator, driving the excessive cell growth and movement that define keloid formation.
The study also analyzed data from The Cancer Genome Atlas (TCGA). This analysis revealed that CLOCK and its regulated genes were upregulated in skin cutaneous melanoma (a type of skin cancer). The upregulation was even greater in metastatic tumors (cancer that has spread). This link suggests that CLOCK is involved in a shared biological pathway for both aggressive cancer growth and excessive scar formation.
In summary, this research is a major advance in understanding fibrotic skin diseases. By dissecting the cellular complexity with scRNA-seq and identifying CLOCK as the key regulator in keloid development, the study opens exciting new treatment possibilities. Targeting the CLOCK pathway could create personalized therapeutic strategies. These strategies could specifically stop the excessive cell proliferation and ECM production, offering hope for millions with severe scarring.
Reference:
NATURE PUBLICATION