Bioenergetics is the study of energy flow through living systems. The production of ATP via Oxidative Phosphorylation is the cornerstone of aerobic life, linking the fundamental laws of thermodynamics to the cellular machinery found in the mitochondria.
1. The Laws of Thermodynamics in Bioenergetics
The principles governing all energy transformations are key to understanding metabolism.
| Law | Principle | Significance in Biology |
| First Law | Conservation of Energy: Energy is neither created nor destroyed; it only changes form. | Cells convert chemical energy from food (e.g., glucose) into mechanical work, chemical bonds, and heat. |
| Second Law | Increase in Entropy: In every energy transfer, the total disorder (entropy, ΔS) of the universe increases. | Living cells maintain a high degree of internal order (low entropy) by constantly increasing the entropy of their surroundings (releasing heat, breaking down complex molecules). |
Export to Sheets
2. Key Thermodynamic Parameters
Gibbs Free Energy (ΔG)
ΔG predicts the spontaneity of a reaction. Cells harness ΔG to drive essential metabolic processes.
- Exergonic Reaction: ΔG<0. Energy is released; the reaction is spontaneous.
- Endergonic Reaction: ΔG>0. Energy is required; the reaction is non-spontaneous.
Enthalpy (ΔH) and Entropy (ΔS)
- Enthalpy (ΔH): The total heat content of a system. ΔH<0 for exothermic (heat-releasing) reactions; ΔH>0 for endothermic (heat-absorbing) reactions.
- Entropy (ΔS): The measure of randomness or disorder. Spontaneous processes favor an increase in disorder (ΔS>0).
ΔG=ΔH−TΔS
3. The Mitochondrial Stage: Electron Transport and Phosphorylation
Oxidative Phosphorylation (OxPhos) is the process where ATP is synthesized using the energy released from the oxidation of nutrients. It is catalyzed by the machinery within the mitochondria.
Mitochondrial Structure ShutterstockExplore
ShutterstockExplore
- Outer Membrane: Highly porous and permeable to small ions and molecules.
- Inner Membrane: Highly impermeable and houses the protein complexes of the Electron Transport Chain (ETC) and ATP Synthase.
- Cristae: Folds of the inner membrane that vastly increase the surface area for ATP production.
- Matrix: The space enclosed by the inner membrane where the Citric Acid Cycle occurs.
The Electron Transport Chain (ETC)
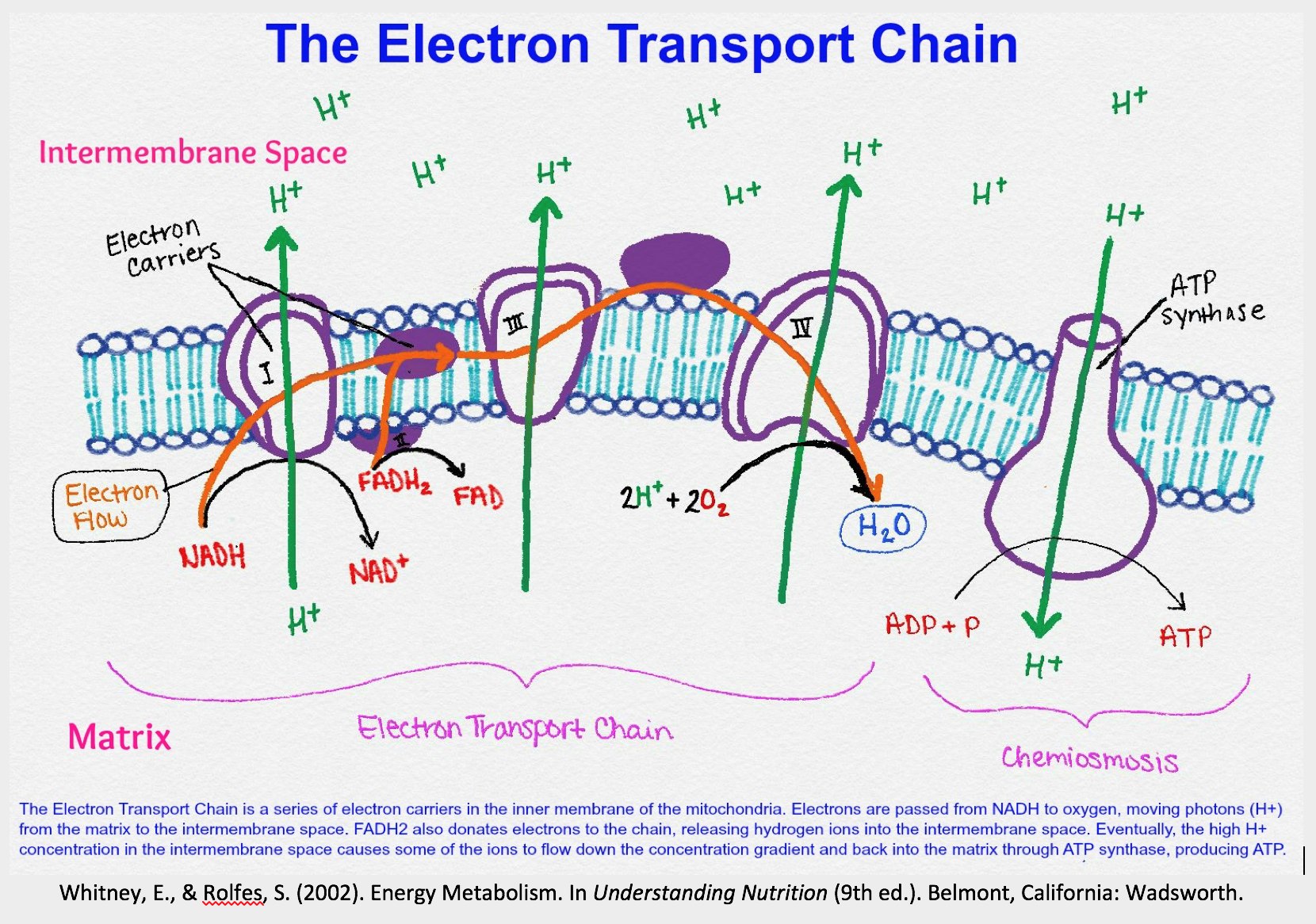
The ETC is a series of four multi-protein complexes (I-IV) embedded in the inner mitochondrial membrane. It accepts high-energy electrons from carriers (NADH and FADH2) generated during glycolysis and the Citric Acid Cycle.
Purpose:
- Transfer electrons from NADH and FADH2 to the final electron acceptor, oxygen (O2).
- Use the energy released from this transfer to pump protons (H+) from the matrix into the intermembrane space, establishing an electrochemical proton gradient.
| Complex | Name | Function | Proton Pumping |
| Complex I | NADH-Q Oxidoreductase | Transfers electrons from NADH to Ubiquinone (CoQ). | Pumps 4 H+ |
| Complex II | Succinate-Q Reductase | Transfers electrons from FADH2 (from succinate oxidation in the CAC) to CoQ. | No H+ Pumping |
| Complex III | Q-Cytochrome c Oxidoreductase | Transfers electrons from CoQH2 to Cytochrome c (mobile carrier). | Pumps 4 H+ |
| Complex IV | Cytochrome c Oxidase | Transfers electrons from Cytochrome c to O2, reducing it to H2O. | Pumps 2 H+ |
Export to Sheets
Mobile Electron Carriers:
- Coenzyme Q (Ubiquinone): A hydrophobic, lipid-soluble carrier that moves within the inner membrane, shuttling electrons from Complexes I and II to Complex III.
- Cytochrome c: A small protein loosely bound to the outer surface of the inner membrane, shuttling electrons from Complex III to Complex IV.
Oxidative Phosphorylation (Chemiosmosis)
This is the final step where ATP is generated. The proton gradient created by the ETC drives the synthesis of ATP.
- Proton-Motive Force: The electrochemical gradient (a combination of a pH difference and an electrical potential) across the inner membrane is called the proton-motive force.
- ATP Synthase: Protons flow back into the matrix down this gradient through the enzyme ATP synthase. The energy of this proton flow causes the rotational mechanism of ATP synthase to catalyze the phosphorylation of ADP to ATP.

ADP+PiATP SynthaseATP+H2O
4. ETC Inhibitors and Uncouplers
Inhibitors
Inhibitors block electron flow at a specific complex, halting the entire ETC and preventing the proton gradient formation, thereby stopping ATP synthesis.
| Inhibitor | Site of Action | Consequence |
| Rotenone, Amytal | Complex I (blocks transfer from NADH to CoQ) | Fatal disruption of energy production. |
| Antimycin A | Complex III (blocks transfer from CoQH2 to Cyt c) | Halts electron flow and oxygen consumption. |
| Cyanide (CN−), Carbon Monoxide (CO) | Complex IV (binds to the active site, preventing O2 reduction) | Rapid cessation of cellular respiration. |
Export to Sheets
Uncoupling Agents
Uncouplers allow the H+ ions to re-enter the mitochondrial matrix without passing through the ATP synthase.
- Mechanism: Chemicals like 2,4-Dinitrophenol (DNP) act as H+ ionophores, creating an alternate path for protons to leak across the inner membrane.
- Consequence: The energy from electron transport is still released, but it is dissipated as heat instead of being conserved as ATP. This process is used physiologically by brown adipose tissue (BAT) to generate heat (thermogenesis).
- Reversibility: The uncoupling process is generally reversible; ATP synthesis resumes when the uncoupler is removed.
Engage with Us:
Stay tuned for more captivating insights and News. Visit our Blogs and Follow Us on social media to never miss an update. Together, let’s unravel the mysteries of the natural world.