The transport of oxygen from the respiratory surfaces to the tissues is a critical physiological process. This article explores the kinetics of hemoglobin saturation, the factors influencing oxygen affinity, and the comparative biochemistry of respiratory pigments across the animal kingdom.
I. The Oxygen-Hemoglobin Dissociation Curve
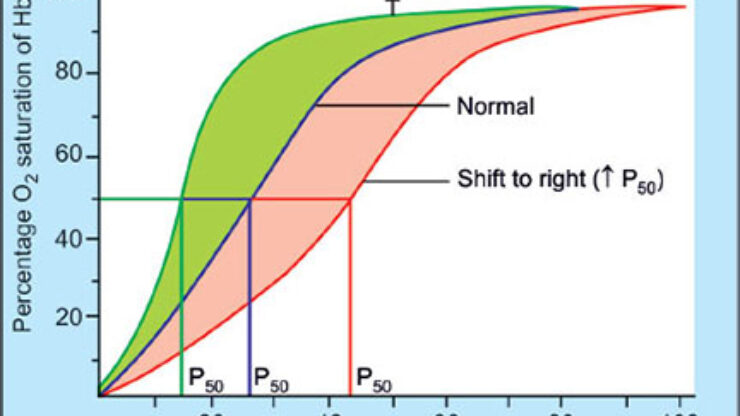
The relationship between the partial pressure of oxygen ($P_{O_2}$) and the percentage saturation of hemoglobin forms a sigmoid (S-shaped) curve. This shape is physiologically significant, reflecting the cooperative binding nature of the hemoglobin molecule.
Nature of the Curve
The curve can be analyzed in three distinct phases:
- Steep Rise (0–40 mmHg): The curve exhibits a steep vertical rise in this range, indicating a rapid increase in oxygen binding. A small increase in $P_{O_2}$ results in a massive increase in hemoglobin saturation.
- Plateau Phase (40–60 mmHg): There is no notable increase in saturation in this range; the curve begins to level off.
- Saturation Phase (60–100 mmHg): The curve becomes nearly parallel to the X-axis. At this stage, hemoglobin is almost fully saturated, and further increases in oxygen tension yield minimal gains in saturation.
Physiological Implication: The sigmoid shape ensures that hemoglobin can load oxygen effectively at the lungs (high $P_{O_2}$) and unload it efficiently at the tissues (low $P_{O_2}$), where high $CO_2$ levels further promote dissociation.
II. Factors Affecting the Dissociation Curve
The affinity of hemoglobin for oxygen is not static; it is modulated by environmental and physiological factors. These shifts are crucial for adaptation in various organisms.
1. Temperature
An increase in temperature creates a “right shift” in the curve, decreasing hemoglobin’s affinity for oxygen.
- Mechanism: Higher temperatures weaken the bond between iron and oxygen.
- Example: At $38^\circ\text{C}$ and a $P_{O_2}$ of 100 mmHg, saturation is lower than it would be at $25^\circ\text{C}$. This allows warm-blooded animals (endotherms) to dissociate oxygen more rapidly to active tissues than cold-blooded animals (ectotherms).
2. Electrolytes
The presence of electrolytes facilitates oxygen release. Pure oxyhemoglobin solutions hold oxygen tighter than blood containing standard electrolytes.
3. The Bohr Effect (pH and $CO_2$)
The Bohr Effect describes the inverse relationship between acidity/$\text{CO}_2$ concentration and oxygen affinity.
- High $CO_2$/Low pH: During intense muscular activity, metabolic waste ($CO_2$ and $H^+$) accumulates. This lowers the pH, causing a rightward shift in the curve. Hemoglobin releases oxygen more readily to the tissues that need it most.
- Physiological Result: In high $CO_2$ environments, oxyhemoglobin formation is inhibited, necessitating increased respiration rates (breathlessness) to maintain energy production.
4. The Root Effect
Observed primarily in fish, the Root Effect is an extreme exaggeration of the Bohr effect.
- Distinction: While the Bohr effect changes affinity, the Root effect reduces the total capacity of hemoglobin to carry oxygen at low pH, even at high oxygen tensions.
III. Carbon Monoxide (CO) Poisoning
Carbon monoxide is a colorless, odorless, and tasteless gas resulting from the incomplete combustion of fuels (gasoline, propane, natural gas). It presents a silent but deadly threat to respiration.
Mechanism of Action
CO enters the bloodstream via the lungs and competes with oxygen for binding sites on the heme group.
$$Hb + CO \rightarrow HbCO \quad (\text{Carboxyhemoglobin})$$
- Affinity: Hemoglobin binds to CO with an affinity approximately 200–250 times higher than its affinity for $O_2$.
- Kinetics: The dissociation constant for CO is extremely low, indicating a very strong bond that is difficult to break. Once CO binds, it blocks oxygen transport and prevents the release of any remaining oxygen to tissues.
Vulnerable Populations: Infants, the elderly, and individuals with anemia or heart disease are at highest risk, particularly during colder months when heating appliances are in heavy use.
IV. Respiratory Pigments
In large multicellular animals, simple diffusion is insufficient for oxygen transport. Respiratory pigments—metalloproteins that reversibly bind oxygen—solve this problem.
There are four primary types of respiratory pigments recognized in the animal kingdom:
1. Hemoglobin
The most efficient and widely distributed pigment.
- Distribution: Found in almost all vertebrates (in erythrocytes) and some invertebrates (often dissolved in plasma, e.g., earthworms).
- Structure: Composed of Heme (a porphyrin ring containing a central ferrous ion, $Fe^{2+}$) and Globin (protein chains).
- Vertebrates: Tetrameric (4 subunits). In adult humans ($HbA$), this consists of $2\alpha$ chains (141 amino acids) and $2\beta$ chains (146 amino acids).
- Invertebrates: often polymeric with high molecular weights (up to 3 million Daltons).
- Reaction: $Hb + 4O_2 \rightleftharpoons Hb(O_2)_4$ (Oxyhemoglobin).
2. Haemocyanin
A copper-containing protein found in Molluscs (snails, octopuses) and Arthropods (crabs, scorpions).
- Characteristics: It is dissolved directly in the plasma and contains no porphyrin group.
- Coloration:
- Deoxygenated: Colorless ($Cu^+$ / Cuprous state).
- Oxygenated: Blue ($Cu^{2+}$ / Cupric state).
3. Chlorocruorin
A green metalloprotein found in the plasma of certain polychaete worms (e.g., Sabellidae).
- Structure: Similar to hemoglobin (contains iron and porphyrin), but the vinyl group on the ring is replaced by a formyl group.
- Color: Green in dilute solutions; light red in concentrated solutions.
4. Hemerythrin
A non-heme iron pigment.
- Distribution: Found in Sipunculids (peanut worms), Priapulids, and Brachiopods.
- Coloration: Colorless when deoxygenated; Violet/Pink when oxygenated.
Summary
The following table summarizes the key distinctions between the major respiratory pigments:
| Feature | Hemoglobin | Haemocyanin | Chlorocruorin | Hemerythrin |
| Metal Core | Iron ($Fe^{2+}$) | Copper ($Cu$) | Iron ($Fe^{2+}$) | Iron ($Fe$) |
| Porphyrin? | Yes (Heme) | No | Yes (Modified Heme) | No |
| Location | Vertebrates (RBCs), Invertebrates (Plasma/Cells) | Arthropods, Molluscs (Plasma) | Polychaete Worms (Plasma) | Sipunculids, Brachiopods (Corpuscles) |
| Color (Oxy) | Red | Blue | Green | Violet |
| Color (Deoxy) | Dark Red / Purple | Colorless | Green | Colorless |
| MW (Daltons) | 64k – 3M | 400k – 13M | ~3M | 100k+ |