Thermodynamics is the branch of science that deals with the relationships between heat, work, and energy. It can be applied to any physical, chemical, or biological system that involves energy transformations.
System and Surroundings
To study thermodynamics, we divide the universe into two parts:
- System: The specific part of the universe we are studying. For example, a glass of hot water.
- Surroundings (or Environment): Everything else outside the system. For example, the room where the glass of water is placed.
The relationship between the system’s temperature (Tsystem) and the surrounding’s temperature (Tenvironment) determines the direction of heat flow:
- If Tsystem>Tenvironment: Heat flows from the system to the surroundings.
- If Tsystem<Tenvironment: Heat flows from the surroundings to the system.
Types of Thermodynamic Systems
Systems are classified based on the exchange of energy and matter with their surroundings:
- Open System: Can exchange both energy and matter with the surroundings.
- Closed System: Can exchange only energy (not matter) with the surroundings. A covered glass of hot water is a good example.
- Isolated System: Cannot exchange either energy or matter with the surroundings.
Equilibrium and Processes
- Thermodynamic Equilibrium: A state where the system’s properties (like temperature) are stable and there is no net flow of energy or matter between the system and its surroundings. This happens when Tsystem=Tenvironment.
- Thermodynamic Process: The path a system takes to change from one state to another.
- Irreversible Process: A spontaneous process that cannot trace back its original path to return to the initial state. Nearly all natural processes are irreversible.
- Reversible Process: A theoretical process that occurs infinitely slowly, with the system always in equilibrium. It can be reversed by an infinitesimally small change.
Heat, Work, and Energy
- Heat (Q): Energy transferred due to a temperature difference.
- Q is positive (+): Heat flows into the system from the surroundings.
- Q is negative (-): Heat flows out of the system to the surroundings.
- Work (W): Energy transferred when a force causes displacement.
- W is positive (+): Work is done by the system.
- W is negative (-): Work is done on the system.
Important Note: Heat and Work are not intrinsic properties of a system; they depend on the process or path taken between states. The conversion factor is 1 calorie = 4.186 joules.
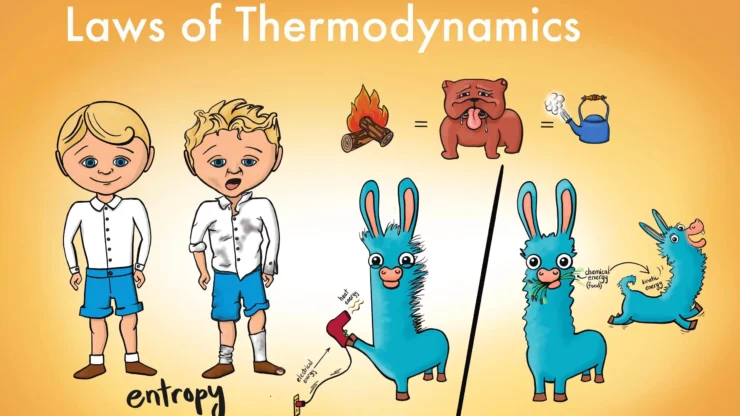
The Laws of Thermodynamics 📜
These are the fundamental principles governing energy transformations.
The First Law of Thermodynamics (Conservation of Energy)
This law states that energy can neither be created nor destroyed, only converted from one form to another.
The mathematical expression is:
dQ−dW=dU
Where:
- dQ is the heat added to the system.
- dW is the work done by the system.
- dU is the change in the internal energy of the system.
While Q and W depend on the path taken, the change in internal energy (dU) is independent of the path and is an intrinsic property of the system.
The Second Law of Thermodynamics (Direction of Processes)
This law establishes the direction in which natural processes occur. It explains why processes are spontaneous and irreversible. It can be stated in several ways:
- It is impossible to convert heat completely into work with no other change taking place.
- It is impossible for heat to flow spontaneously from a colder body to a hotter body.
The Third Law of Thermodynamics (Absolute Zero)
This law deals with the behavior of systems as they approach absolute zero temperature.
- The entropy of a pure, perfectly crystalline solid or liquid is zero at absolute zero (0 Kelvin).
- It is impossible to reach the temperature of absolute zero in a finite number of steps.
Entropy
Entropy (S) is a thermodynamic variable that provides a precise quantitative measure for the second law of thermodynamics. It is defined as a thermodynamic state function, meaning its change between two states is independent of the path taken during the transformation of the system.
For a reversible process, the infinitesimal change in entropy (dS) is defined as the reversible heat transfer (dQrev) divided by the absolute temperature (T) at which the transfer occurs:
dS=dQrev/T
The total change in entropy (ΔS) between an initial state (1) and a final state (2) is found by integrating this expression:
ΔS=S2−S1=∫12dQrev/T
Like temperature (T) and internal energy (U), entropy is a state function; it depends only on the current state of the system, not on how it arrived there. While the entropy change for a reversible process can be calculated directly, the change for an irreversible process is determined by devising a reversible path that connects the same initial and final states.
The second law of thermodynamics can be stated in terms of total entropy change:
- For an irreversible process in an isolated system: ΔStotal>0
- For a reversible process in an isolated system: ΔStotal=0
This implies that for all natural, spontaneous processes, including biological reactions, the total entropy of the universe always increases.
Ludwig Boltzmann provided a statistical interpretation of entropy, relating it to molecular disorder with the equation:
S=klnW
Here, k is Boltzmann’s constant, and W is the number of possible microscopic arrangements (microstates) of the atoms or molecules in the system that correspond to the observed macroscopic state. A higher value of W signifies a more disordered system. Therefore, an increase in entropy corresponds to an increase in randomness or disorder.
Enthalpy
Enthalpy (H) is a thermodynamic potential defined as the sum of a system’s internal energy (U) and the product of its pressure (P) and volume (V):
H=U+PV
The term PV represents the energy required to make room for the system by displacing its environment. Since enthalpy is composed of state functions (U, P, and V), it is also a state function, and its change is path-independent. Enthalpy is often referred to as the “heat content” of the system.
The differential form of the enthalpy equation is:
dH=dU+PdV+VdP
For a process occurring at constant pressure (dP=0), this simplifies to:
dH=dU+PdV
According to the first law of thermodynamics, dU=dQ−PdV. Substituting this into the equation above yields:
dH=(dQ−PdV)+PdV
dH=dQP
This crucial result shows that at constant pressure, the change in enthalpy is exactly equal to the heat (QP) absorbed or released by the system.
- An exothermic process releases heat, so ΔH is negative.
- An endothermic process absorbs heat, so ΔH is positive.
Free Energy of a System
New state functions, known as thermodynamic potentials, can be created through linear combinations of fundamental state functions like internal energy and entropy. Enthalpy, Helmholtz free energy, and Gibbs free energy are the most important thermodynamic potentials.
(a) Helmholtz Free Energy (F)
The Helmholtz free energy (F) is defined as:
F=U−TS
Its differential form is dF=dU−TdS−SdT. Since the first law can be written as dU=TdS−PdV (for a reversible process), we can substitute this to get:
dF=(TdS−PdV)−TdS−SdT
dF=−PdV−SdT
For a process occurring at constant temperature (dT=0), the change in Helmholtz free energy is dF=−PdV. This means that under isothermal, reversible conditions, the work done by the system is equal to the decrease in its Helmholtz free energy. A spontaneous process at constant temperature and volume proceeds in the direction of decreasing Helmholtz free energy, reaching a minimum at equilibrium.
(b) Gibbs Free Energy (G)
The Gibbs free energy (G) is the most chemically relevant thermodynamic potential, defined as:
G=H−TS=U+PV−TS
Its differential form is dG=dH−TdS−SdT. We know that for a reversible process, dH=TdS+VdP. Substituting this gives:
dG=(TdS+VdP)−TdS−SdT
dG=VdP−SdT
For a process occurring at constant pressure and temperature (dP=0 and dT=0), which describes conditions for most biological and chemical reactions, the change in Gibbs free energy is given by the famous Gibbs-Helmholtz equation:
ΔG=ΔH−TΔS
The sign of ΔG predicts the spontaneity of a process under these conditions:
- If ΔG<0: The process is spontaneous in the forward direction.
- If ΔG>0: The process is non-spontaneous (the reverse process is spontaneous).
- If ΔG=0: The system is at equilibrium.
Thus, Gibbs free energy represents the maximum amount of non-expansion work that can be extracted from a system at constant temperature and pressure. At equilibrium, the system is in its most stable state, where ΔG=0.